Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Watanabe, Satoshi; Ishioka, Noriko; Osa, Akihiko; Koizumi, Mitsuo; Sekine, Toshiaki; Kiyomiya, Shoichiro*; Nakanishi, Hiromi*; Mori, Satoshi*
Radiochimica Acta, 89(11-12), p.853 - 858, 2002/02
Times Cited Count:17 Percentile:74.85(Chemistry, Inorganic & Nuclear)no abstracts in English
Osaka, Masahiko; Koyama, Shinichi; Mitsugashira, Toshiaki; Morozumi, Katsufumi; Namekawa, Takashi
JNC TN9400 2000-058, 49 Pages, 2000/04
The analytical technique for Cm contained in a MOX FUEL was developed and analysis of Cm contained in irradiated fuel of experimental fast reactor "JOYO" was carried out, to contribute to evaluation of transmutation characteristics of MA nuclide in the fast reactor. The procedure of ion-exchange separation of Cm with nitric acid-methanol mixed media essential for the isotopic analysis in irradiated MOX fuel was adopted considering for being rapid and easy. The fundamental test to grasp separation characteristics of this procedure, such as Cm elution position and separation capacity between Cm and Am or Eu, was carried out. ln applying this procedure to the analysis of Cm contained in actual specimen, separation condition was evaluated and optimized, and the procedure consist of impurity removal and Am removal process was devised. This procedure resulted in high recovery rate of Cm and high removal rate of Am and impurity which becomes a problem in sample handling and mass-spectrometry such as Eu and Cs. The Cm separation test from irradiated MOX fuel was carried out using this technique, and Cm isotopic ratio analysis was enabled. The analytical technique for Cm contained in irradiated MOX fuel was established using the procedure of ion-exchange separation with nitric acid-methanol mixed media. The analysis of Cm contained in irradiated MOX fuel of experimental fast reactor "Joyo" was carried out. As a result, it was revealed from measured data that Cm content rate was 1.4 4.0
4.0 lO
lO atom%, small amount of
atom%, small amount of 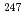 Cm was generated and Cm isotopic ratio was constant above burn-up 60GWd/t.
Cm was generated and Cm isotopic ratio was constant above burn-up 60GWd/t.
*; Arai, Tsuyoshi*; Kumagai, Mikio*
JNC TJ9400 2000-002, 80 Pages, 2000/02
In order to develop an economically efficient wet separation process other than solvent extraction for reprocessing spent FBR-fuel (MOX fuel), we have investigated the possibility of an advanced ion exchange process. Based on the fundamental research results, we proposed an advanced ion exchange process considering the characteristics of FBR-fuel cycle. The separation system consists of a main separation process using a novel anion exchanger which has a rapid kinetics and two extraction chromatography processes for minor actinides isolation using novel impregnation adsorbents with high selectivity. The chemical flow sheet, mass balance chart, list of main equipment and installation layout of each equipment were estimated and designed for the process in a reprocessing plant with the capacity of 200 tHM/y FBR-fuel. The process was pfeliminarny evalualed from the aspects of economy performance, recovery of potentially useable resources, minimization of environmental risk and proliferation-resistance by comparing with the advanced PUREX process. Furthermore, the subjects which are important for the practical application of the process are also listed.
*; *; Fumoto, Hiromichi*; *; *
JNC TJ9400 2000-001, 112 Pages, 2000/02
The purpose of this study is to investigate the possibility of new reprocessing process for the purpose of introducing pipeless plant concept, where aqueous separation methods other than solvent extraction method are adopted in order to develop more economical FBR fuel (MOX fuel) reprocessing process. At it's first stage, literature survey on precipitation method, crystallization method and ion-exchange method was performed. Based on the results, following processes were candidated for pipeless reprocessing plant. (1)The process adopting crystallization method and peroxide precipitation method (2)The process adopting oxalate precipitation method (3)The process under mild aqueous conditions (crystallization method and precipitation method) (4)The process adopting crystallization method and ion-exchange method (5)The process adopting crystallization method and solvent extraction method The processes (1) (5) were compared with each others in terms of competitiveness to the conventional reference process, and merits and demerits were evaluated from the viewpoint of applicability to pipeless reprocessing plant, safety, economy, Efficiencies in consumption of Resources, non-proliferation, and, Operation and Maintenance. As a result, (1)The process adopting crystallization method and peroxide precipitation method was selected as the most reasonable process to pipeless plant. Preliminary criticality safety analyses, main process chemical flowsheet, main equipment list and layout of mobile vessels and stations were reported for the (1) process.
(5) were compared with each others in terms of competitiveness to the conventional reference process, and merits and demerits were evaluated from the viewpoint of applicability to pipeless reprocessing plant, safety, economy, Efficiencies in consumption of Resources, non-proliferation, and, Operation and Maintenance. As a result, (1)The process adopting crystallization method and peroxide precipitation method was selected as the most reasonable process to pipeless plant. Preliminary criticality safety analyses, main process chemical flowsheet, main equipment list and layout of mobile vessels and stations were reported for the (1) process.
Tsukada, Kazuaki
Kagaku To Kogyo, 51(4), P. 615, 1998/00
no abstracts in English
Sumiya, Shuichi; Hayashi, Naomi; ; Narita, Osamu
PNC TN8430 91-001, 45 Pages, 1990/12
A radioanalytical method for low level samarium-151(Sm-151) and promethium-147(Pm-147) in environmental samples has been studied for the environmental assessment around nuclear facilities. In this study, we use the separation method with high performance liquid chromatography (HPLC) to determine Sm-151 and Pm-147 in environmental samples such as sea sediments and marine organisms. Samarium-151 and Pm-147 in environmental samples are coprecipitated with other lanthanoids after adding neodymium(Nd). These nuclides are purified by anion exchange methods in methanol-mineral acid media. After the purification, Sm-151 and Pm-147 are separated with HPLC in lactic acid-sodium hydroxide media, and determined with liquid scintillation counting, respectively. The Nd is determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) to correct chemical recoveries of these nuclides. The detection limits for Sm-151 and Pm-147 in this method are about 0.01Bq/sample.
JAERI-M 8339, 89 Pages, 1979/07
no abstracts in English
 O
O
;
Journal of Nuclear Science and Technology, 14(5), p.376 - 380, 1977/05
Times Cited Count:2no abstracts in English
; ; ; ; ; ;
JAERI-M 6958, 40 Pages, 1977/03
no abstracts in English


 Pu
Pu; ; Umezawa, Hirokazu; ; *
Int.J.Appl.Radiat.Isot., 27(12), p.713 - 715, 1976/12
Times Cited Count:10no abstracts in English
Journal of Nuclear Science and Technology, 13(8), p.449 - 453, 1976/08
Times Cited Count:1no abstracts in English
;
Journal of Inorganic and Nuclear Chemistry, 35(7), p.2592 - 2594, 1973/07
Times Cited Count:5no abstracts in English
Nihon Genshiryoku Gakkai-Shi, 11(11), p.687 - 690, 1969/00
no abstracts in English

 S by the neutron irradiation on potassium chloride; Separation of
S by the neutron irradiation on potassium chloride; Separation of 
 S by the combined method of precipitation and anion exchange
S by the combined method of precipitation and anion exchangeNihon Genshiryoku Gakkai-Shi, 5(8), p.644 - 651, 1963/00
no abstracts in English
Nihon Genshiryoku Gakkai-Shi, 4(3), p.154 - 160, 1962/00
no abstracts in English
; ; ; ; *; *; *; *
Nihon Genshiryoku Gakkai-Shi, 4(10), p.700 - 702, 1962/00
no abstracts in English
Takahatake, Yoko; Saito, Madoka*; Iwamoto, Toshihiro; Watanabe, So; Watanabe, Masayuki
no journal, ,
The sludge contained uranium generated production of nuclear fuel has been storage. The sludge is immersed in some kinds of solution. After immersion, uranium is recovered from the solution. Survey of uranium recovery methods was conducted for realization of technical scale facility which is treated sludge solution. Result of comparison on facility scale, amount of second waste, maturity, merit and demerit, several methods which have to be considered were selected.